In this application, SR-FTIR spectromicroscopy was used to accelerate the process of engineering and optimizing the cyanobacterial conversion of CO2 to high-density liquid fuels. Cyanobacteria, like algae and plants can use solar power to capture CO2 via the Calvin-Benson-Bassham (CBB) cycle and convert it to a suite of organic compounds.
Cyanobacteria are Gram-negative bacteria and are well suited for synthetic biology and metabolic engineering approaches for the phototrophic production of various desirable biomolecules, including ethanol, butanol, biodiesel, and hydrocarbon biofuels. Phototrophic biosynthesis of high-density liquid biofuels in cyanobacteria would serve as a nice complement to the microbial production of biodiesel and hydrocarbons in heterotrophic bacteria such as E. coli.
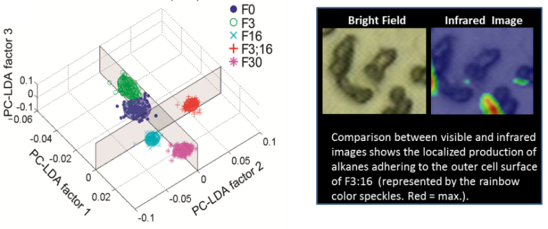
To evaluate this possibility, Jansson and his team constructed strains of the cyanobacterium Synechocystis 6803 with altered metabolic traits affecting the acyl-CoA pool: F3, F16, F3:16, and F30, with F0 as the wildtype control. The metabolic responses to the modified traits were interrogated by single-cell metabolic phenotyping in real time by synchrotron radiation Fourier transform infrared (SR-FTIR) spectromicroscopy and multivariate analysis, followed by bulk metabolic assays using GC/MS and NMR.
A strain with extra copies of the FAR and FAD genes, encoding, respectively, the fatty acyl-ACP reductase and fatty aldehyde decarbonylase enzymes in the alkane biosynthesis pathway, showed an up to five-fold increase in the intracellular levels of heptadecane, a three-fold increase in 9-heptadecene, and a significant increase in secreted 16:0 and 18:0 free fatty acids (FFAs). Inactivation of the AAS gene, encoding acyl-ACP synthetase, prevented re-thioesterification of FFAs generated from membrane lipid recycling and led to elevated levels and an altered composition of intracellular FFAs, and a decrease in heptadecane and secreted FFAs.
Introduction of a FatB gene, encoding a thioesterase (TE), which catalyzes the liberation of FFAs from acyl-ACP, yielded little effect in itself but the activity of the TE enzyme was clearly manifested in combination with AAS inactivation; A TE-containing strain with inactivated AAS showed a dramatic (30-fold) increase in intracellular FFAs with the majority being 16:0, and an increase in heptadecane and in secreted FFAs. SR-FTIR difference spectra and cluster vector plots of the strains were consistent with the GC/MS/NMR data.
Furthermore, they demonstrated that elevated levels of carbonyl groups in the TE-containing/AAS-lacking strain could be attributed to carboxyl ester bonds in polyhydroxyalkanoate (PHA), implying biosynthesis of the storage compound polyhydyroxybutyrate (PHB).